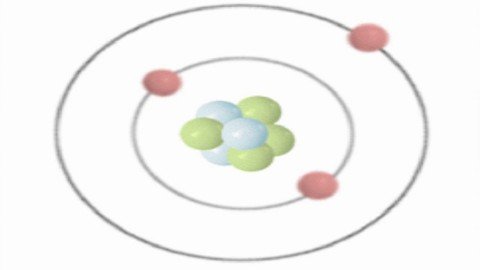
Learn Chemistry - Structure Of Atoms
"softddl.org"
19-07-2022, 13:04
-
Share on social networks:
-
Download for free: Learn
-
Last updated 10/2021
MP4 | Video: h264, 1280x720 | Audio: AAC, 44.1 KHz
Language: English | Size: 742.95 MB | Duration: 2h 24m
Atomic Structure

Last updated 10/2021
MP4 | Video: h264, 1280x720 | Audio: AAC, 44.1 KHz
Language: English | Size: 742.95 MB | Duration: 2h 24m
Atomic Structure
What you'll learn
Basic Concepts of Chemistry - Structure of Atoms
Detailed explanation with examples
Easy and understandable language to get exact knowledge
Practice questions and quiz for better understanding
Requirements
No skills required to understand the course. All the concepts will be explained in course.
Description
The course structure of atoms covers the study of the most important part of complete chemistry i.e Atom. In this course, you will get to learn how the subatomic particles were discovered and how the generations of scientific work ultimately came together to create our understanding of the most fundamental unit of matter i.e, atoms. Also here we will discuss the electronic and structural properties of atoms, the relationship between the mass number of an atom, its atomic number, its atomic mass, and its no. of subatomic particles. Here in this course, you will get to learn how to solve the numerical problems on all these concepts and there are quizzes to check your understanding of each concept.The course content is as follows : Sub-atomic Particles, Discovery of Electrons, Discovery of Protons & Neutrons, Atomic Models, Atomic Number and Mass Number, Isotopes & Isobars, Wave Nature of Electromagnetic Theory, Characteristics of Waves, Spectrum of electromagnetic radiation, Black body radiation, Plank's Quantum theory, Photo electric effects, Spectrum, Line spectrum of hydrogen, Bohr's model of atom, De Broglie's equation, Heisenberg's uncertainty principle, Quantum mechanical model of atom, Quantum number, Shape of S-orbital, Shape of P-orbital, Shape of D-orbital etc. along with quiz.Targeted Audience :For XI, XII (All boards like CBSE, ICSE, IB, State board etc)For IIT-JEE and NEET ExamsFor Competitive ExamsAnyone who wants to enhance their knowledge.
Overview
Section 1: Introduction
Lecture 1 1. Introduction of Structure of Atoms
Lecture 2 2. Sub-atomic Particles
Lecture 3 3. Discovery of Electrons
Lecture 4 4. Charge to Mass Ratio of Electrons
Lecture 5 5. Discovery of Protons & Neutrons
Section 2: Atomic Model
Lecture 6 6. Atomic Model
Lecture 7 7. Thomson's Model of Atom
Lecture 8 8. Rutherford's Model of Atom
Lecture 9 9. Atomic Number and Mass Number
Lecture 10 10. Isotopes & Isobars
Lecture 11 11. Drawbacks of Rutherford's Model
Lecture 12 12. Developments Leading to Bohr's Model of Atom
Section 3: Waves & Waves Theory
Lecture 13 13. Wave Nature of Electromagnetic Theory
Lecture 14 14. Characteristics of Waves
Lecture 15 15. Spectrum of Electromagnetic Radiation
Lecture 16 16. Black Body Radiation
Lecture 17 17. Plank's Quantum Theory
Lecture 18 18. Photoelectric Effect
Lecture 19 19. Dual Behavior of Electromagnetic Radiation
Section 4: Model of Atom
Lecture 20 20. Spectrum
Lecture 21 21. Line Spectrum of Hydrogen
Lecture 22 22. Bohr's Model of Atom
Lecture 23 23. De Broglie's Equation
Lecture 24 24. Heisenberg's Uncertainty Principle
Lecture 25 25. Failure of Bohr's Model
Lecture 26 26. Quantum Mechanical Model of Atom
Section 5: Quantum Number
Lecture 27 27. Quantum Number
Lecture 28 28. Principle Quantum Number
Lecture 29 29. Azimuthal Quantum Number
Lecture 30 30. Magnetic Quantum Number
Lecture 31 31. Spin Quantum Number
Lecture 32 32. Shape of S-orbital
Lecture 33 33. Shape of P-orbital
Lecture 34 34. Shape of D-orbital
Lecture 35 35. Aufbau's Principle
Lecture 36 36. Pauli's Exclusion Principle
Lecture 37 37. Hund's Rule of Maximum Multiplicity
Lecture 38 38. Stability of Orbitals
Section 6: Numerical
Lecture 39 39. Numerical Problem
Lecture 40 40. Quiz
For academic purpose like school students, college students, competitive exams aspirants etc.
Homepage
https://www.udemy.com/course/learn-chemistry-structure-of-atoms/Links are Interchangeable - No Password - Single Extraction
The minimum comment length is 50 characters. comments are moderated